
The entropy data are therefore given as absolute numbers, S o, not entropies of formation, S o f.The second law says that the entropy change must be equal to orgreater than zero. As a result, the absolute entropy of any element or compound can be measured by comparing it with a perfect crystal at absolute zero. The third law defines absolute zero on the entropy scale. Entropy data are different.
We have seen that the energy given off (or.This page deals only with entropy changes to the system. Calculation of Entropy Changes. For a given physical process, the combined entropy of the system and the environment remains a constant if the process can be reversed.Note: If you haven't already read the page about introducing entropy, you should do so before you go on.CHM 1046. The change in entropy delta S is equal to the heat transfer delta Q divided by the temperature T. This is one of the statements of the second law given inSection 5.1.This page looks at how you can calculate entropy changes during reactions from given values of entropy for each of the substances taking part.The second law states that there exists a useful state variable called entropy S.
...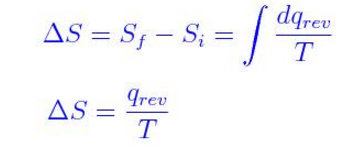
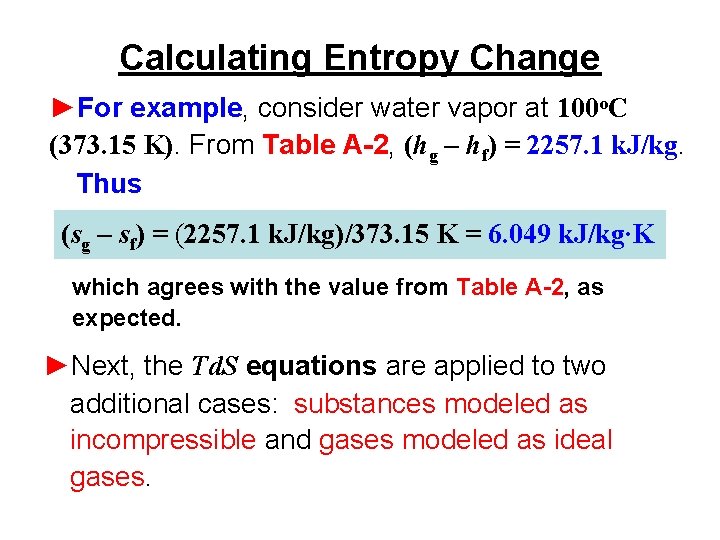
Entropy Change Formula Free EnergyTo The
For the purposes of this page, you can ignore any reference to the word "system".Because this is all covered in detail in my calculations book I shan't be setting any questions throughout this section on entropy and free energyTo the entropy and free energy menu.


 0 kommentar(er)
0 kommentar(er)
